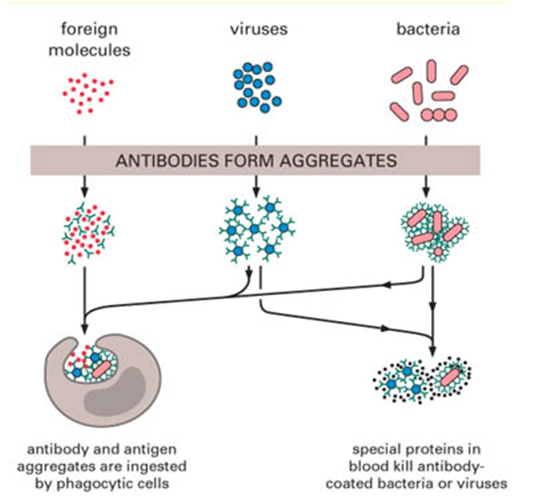
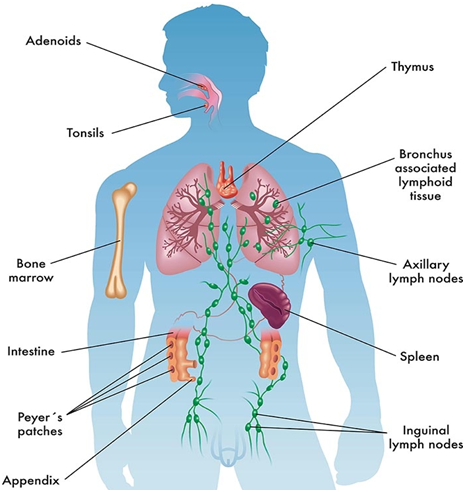
B cells are specialized type of lymphocytes that are responsible for the production of antibodies or immunoglobulins that act as effector molecules to stimulate the entire immune system against an invading antigen or pathogen. They are primarily responsible for the development of antibody mediated immunity (AMI) in the body; and B cells are produced in the bone marrow. In birds, B cells are produced in the Bursa of Fabricius, a lymphoid organ situated near the terminal end of the gut in birds or fowl (i.e. around the cloacae region).
The removal of Bursa of Fabricius from chicken or birds (a medical procedure known as bursectomy) will impair antibody mediated immune response in the chicken because the affected birds will no longer secrete B cells which are biologically significant for the production of antibodies and memory B cells during the invasion of pathogens. This also applies to mouse whose thymus is removed through thymectomy.
Thymectomy is the medical procedure of removing the thymus through surgery; and this procedure exposes the animal to incessant attacks from pathogens especially intracellular parasites. Such mice would find it difficult to produce effector T lymphocytes because the thymus is the organ where T cells mature after their production in the bone marrow. Mice whose thymuses have been surgically removed will generally have an incapacitated cell-mediated immune response especially to intracellular pathogens including viruses which are known to reside within their host cells.
The bone marrow is the site where all the cells of the immune system (e.g. B and T lymphocytes) inclusive of blood cells (e.g. red blood cells, white blood cells and platelets) are initially derived from. They are derived from haematopoietic stem cells (HSCs) during haematopoiesis in the bone marrow.
Stem cells are self-renewing cells found in the bone marrow and which undergo cell division to differentiate and proliferate into other important cells inclusive of the immune system cells and blood cells. HSCs are pluripotent or multipotent in nature because they can differentiate into a wide variety of other cells (e.g. lymphocytes, erythrocytes). B cell maturation is a continuous process that occurs throughout life in the bone marrow of adults.
However, the process of B cell maturation in neonates or fetus in utero (i.e. before birth) usually occurs in the fetal bone marrow, yolk sac or fetal liver; and after birth B cell maturation continues in the bone marrow. After production in the bone marrow, the B cells migrate to secondary lymphoid organs such as the lymph nodes where they differentiate into plasma cells that mediate or stimulate antibody production but only in the appearance of an antigen.
Apart from differentiating into plasma cells for antibody production; the B cells are also responsible for the presentation of antigens to T helper cells which facilitate cell-mediated immunity (CMI) in the host. Thus B cells can act as both antigen presenting cells (APCs) and synthesizing machinery for antibodies and memory cells.
T cells as shall be seen later only recognize antigen molecules presented in association with major histocompatibility complex (MHC) molecules. Nevertheless B cells can recognize antigen molecules by itself and without the assistance of MHC molecules; and this is a major difference between B cells and T cells. T cell maturation occurs in the thymus; and its activation and differentiation occurs in the peripheral lymphoid tissue (e.g. spleen) after encountering an antigen presented by MHC molecule(s).
The activation of B cells is driven by the introduction of foreign bodies or antigens into the body; and once activated; B cells undergo proliferation and differentiation into numerous plasma cells for the production of abundant antibodies with unique specificity for antigens. During differentiation or proliferation, the B cells mainly develop into two types of cells viz: memory cells and plasma cells.
While plasma cells are largely responsible for the production of antibodies and other effector molecules; the memory cells or memory B cells remain in the circulating blood or body fluids after the primary immune response to police the body and eliminate the invading antigen should the body become exposed to a previously exposed antigen or foreign body the second time (i.e. in the future); and this usually happens during a secondary immune response in which the immune system mounts a rapid attack against the invading pathogen. The stages involved in the production of immunocompetent B cells in the bone marrow are a complex set of biological process (Figure 1). However, these stages involved are as follows:
- Maturation: At maturation stage, immunocompetent B cells are produced in the bone marrow by competent progenitor stem cells.
- Activation: Once produced, the immunocompetent B cells become activated following their interaction with antigens or invasion of antigens.
- Differentiation: After the interaction of the immunocompetent B cells with the antigen(s), the activated B lymphocytes differentiate into plasma and memory B cells.
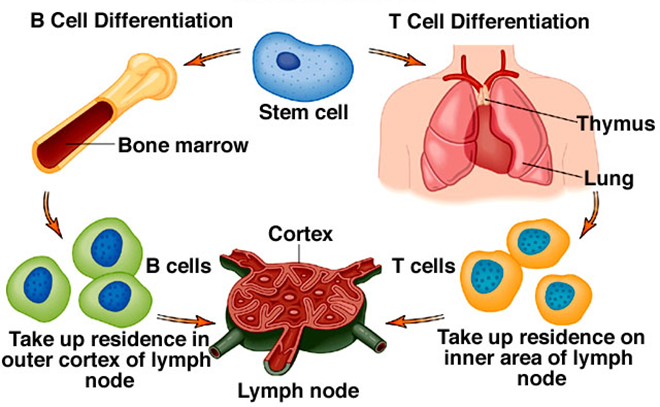
The initial stage of B cell development in the bone marrow (known as antigen-independent stage) is carried out without the presence of any stimulating antigen or foreign body; and the B lymphocytes so produced are generally known as incompetent B cells because they have not encountered any antigen. Such B cells only circulate in the blood before being transported to the lymph nodes or spleen until a foreign body is encountered in the body. However, upon encountering an antigen, the B cells become activated and proliferate in order to differentiate into numerous plasma cells (for antibody production) and memory B cells for immunological memory should incase the antigen invades the body the second time in the future.
The proliferation of B cells at this stage into more B lymphocytes for the production of antibody-producing plasma cells and memory B cells is generally known as clonal expansion/selection. In clonal expansion/selection, a particular B or T cell undergo cell division and differentiate into clones of B and T cells respectively with unique antigenic specificity as that of the original B or T cell that they arise from.
Clonal expansion is the enlargement in the number of competent B cells or T cells with the same antigenic specificity as the progenitor (parent) B cell or T cell. Such B or T cells arise from the same clone of B or T lymphocytes. The expansion of B and T cells in this fashion is controlled by the clonal selection theory. This later stage of B cell development is known as the antigen–dependent stage because the activation and differentiation of B cells require a prior exposure to antigens.
B cells have very short lifespan (about 8 weeks) and they die-off if they fail to encounter an antigen; and thus B lymphocytes circulate as incompetent B cells and compete amongst themselves for space in the secondary lymphoid organs (e.g. spleen) where they remain for the invasion of foreign bodies or antigens. The naïve or incompetent B cells that die-off because they did not encounter antigen for activation and proliferation die through a biological process known as apoptosis (i.e. a programmed cell death.
The formation of specific antibodies each and every time an antigen invades the host is mainly directed or mediated by the prior interaction of the foreign body with antibody-secreting B cells. It is noteworthy that the B lymphocytes are saddled with surface receptors e.g. membrane-bound IgD (mIgD) or membrane-bound IgM (mIgM) that react specifically with the invading antigen. After this interaction between the antigen and the immunologically responsive B cells, the B lymphocytes proliferate into clones of B cells that produce numerous antibody-secreting plasma cells that secrete immunoglobulins that are specific for the antigens.
A clone is a cluster of cells that arise from a single progenitor cell. This mechanism through which antibody-secreting plasma cells emanate from a collection of activated or immunologically responsive B lymphocytes is known as clonal selection; and clonal selection is largely responsible for the secretion of numerous antibodies with specificity for unique antigenic determinant sites or epitopes of antigens.
Clonal selection as earlier stated is the immunological mechanism in which antigen-binding receptors of B cells and T cells stimulate the lymphocytes (i.e. B and T cells) to proliferate into a clone of lymphocytes that produce effector cells (e.g. antibodies, T helper cells and T cytotoxic cells) with the same antigenic specificity as their progenitor or parent cell. It is naïve or immunocompetent B cells that become activated via antigen-antibody reaction, and then differentiate or proliferate into numerous antibody-secreting plasma cells and memory B cells.
The number of B or T cells produced during clonal selection/expansion is amplified; and the process ensures that sufficient amount of memory lymphocytes (B and T cells inclusive) with unique antigenic specificity are always available to assuage the activities of invading pathogens or antigens in the body. Aside the secreted antibodies, the immunologically responsive B cells also proliferate into memory B cells which remain dormant but viable in the blood circulation until a later time when similar antigen invades the host’s body again; and memory B cells explain the reason for the rapid response of antibody production in secondary immune response.
Effector cells of the immune system are immunologically responsive cells that directly encounter antigens and facilitate their neutralization and possible elimination from the host. Examples of effector cells include plasma cells and T cells. Plasma cells are the effector cells of the humoral or antibody-mediated immunity mainly responsible for antibody secretion while the effector cells of the T cells are T helper cells (CD4+) and T cytotoxic cells (CD8+). Self-reactive T lymphocytes (i.e. T cells that attacked host cells instead of antigens) are eliminated from the thymus through the process of clonal deletion.
In summary, the proliferation of B cells and T cells into effector cells is largely governed by the clonal selection theory; and this theory postulates that clones of effector B and T cells arise from a single parent cell that is stimulated via antigen-binding to reproduce similar lymphocytes with unique immunologic specificity and memory.
The clonal selection theory postulates that all the antibody molecules on a single B cell (i.e. antibodies that originated from a single progenitor or parent B cell) have the same antigenic specificity as their parent B cell; and this also apply to the differentiation of naïve T cells into immunocompetent T cells with antigenic specificity as that of their parent T cell. It is a biological theory that explains the unique specificity of antibody formation; and the clonal selection theory is generally based on the certainty that each lymphocyte is programmed to produce a particular type of immunoglobulin that is eventually selected by prior contact with an antigen or pathogen.
All the B and T cells in the expanded parent clones are specific for the epitopes of the original pathogen or antigen that activated their production and selection/expansion into effector cells (i.e. memory B cells, memory T cells and immunocompetent B and T cells). The clonal expansion of T cells occurs after the T cells have encountered an antigen presented by any of the MHC molecules; and the T cells (e.g. CD4+ helper T cells) secrete cytokines which go on to stimulate an antigen-activated B cell to proliferate and differentiate into antibody-secreting plasma cells that eventually produce specific antibodies that bind and facilitates the degradation and removal of the invading pathogen or antigen. This explains how the B cells and T cells cooperatively neutralize the activities of antigens in the body.
REFERENCES
Abbas A.K, Lichtman A.H and Pillai S (2010). Cellular and Molecular Immunology. Sixth edition. Saunders Elsevier Inc, USA.
Actor J (2014). Introductory Immunology. First edition. Academic Press, USA.
Alberts B, Bray D, Johnson A, Lewis J, Raff M, Roberts K and Walter P (1998). Essential Cell Biology: An Introduction to the Molecular Biology of the Cell. Third edition. Garland Publishing Inc., New York.
Bach F and Sachs D (1987). Transplantation immunology. N. Engl. J. Med. 317(8):402-409.
Barrett J.T (1998). Microbiology and Immunology Concepts. Philadelphia, PA: Lippincott-Raven Publishers. USA.
Jaypal V (2007). Fundamentals of Medical Immunology. First edition. Jaypee Brothers Medical Publishers (P) Ltd, New Delhi, India.
John T.J and Samuel R (2000). Herd Immunity and Herd Effect: New Insights and Definitions. European Journal of Epidemiology, 16:601-606.
Levinson W (2010). Review of Medical Microbiology and Immunology. Twelfth edition. The McGraw-Hill Companies, USA.
Roitt I, Brostoff J and Male D (2001). Immunology. Sixth edition. Harcourt Publishers Limited, Spain.
Zon LI (1995). Developmental biology of hematopoiesis. Blood, 86(8): 2876–91.
Discover more from Microbiology Class
Subscribe to get the latest posts sent to your email.