Antibodies are soluble protein molecules produced by the B cells of the immune system in response to a specific antigen. They are antigen recognition molecules; and antibodies generally recognize and bind to specific antigens. The term antibodies is used synonymously with immunoglobulins (Ig).
Antibodies are glycoproteins that are produced by B cells (specifically plasma cells) in response to an immunogenic substance. After production by the B cells, antibodies continue to circulate in the bloodstream as effector cells of the antibody mediated immunity (AMI). Other antigen recognition molecules include major histocompatibility complex (MHC) molecules, T cell receptors, and the B cell receptors.
The production of antibodies is stimulated foreign bodies or antigens that enter the body. Immunoglobulins or globulin proteins are mainly found in blood plasma where they unleash their immunological attack in the face of an antigen.
Antibodies perform various functions in the body and these functions are as follows:
- Antibodies act as opsonins to facilitate the process of phagocytosis. This process is known as opsonization; and it is the deposition of opsonins on the surfaces of pathogenic microorganisms or antigens so that they can be readily phagocytosed by phagocytic cells.
- Antibodies known as agglutinins react specifically with antigens to form clumps in the process of agglutination. This is the basis for most of the in vitro latex agglutination test performed in the laboratory. Agglutination is the visible clumping of particulate antigens by antibodies.
- Antibodies help to activate nonspecific immune response against an invading pathogen.
- Antibodies help to activate the complement system after binding with a particular antigen; and this process stimulate opsonization and microbial cell lysis. The complement system is a system that consists of a series of serum proteins that is activated by antigen-antibody complex to facilitate phagocytosis and other immunological reactions.
- Antibodies serve to mark and identify specific targets for immunological attack in vivo.
- Antibodies search the bloodstream for antigens and neutralize them or mark them for elimination by other cells of the immune system (e.g. phagocytes).
- Antibodies promote antibody-dependent cell-mediated cytotoxicity (ADCC). ADCC is a cell-mediated reaction in which nonspecific cytotoxic cells that expresses Fc regions (i.e. crystallizable fragment portion of an immunoglobulin) such as macrophages and natural killer (NK) cells recognize and bind antibody on a target cell and subsequently cause the lysis of the target cell.
- Antibodies neutralize pathogenic microorganisms (inclusive of bacteria, toxins and viruses) in the process of neutralization.
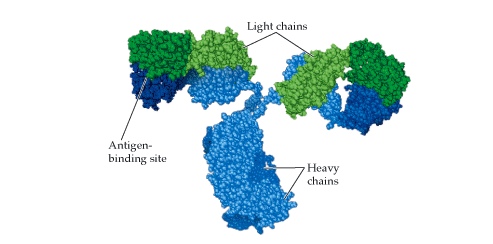
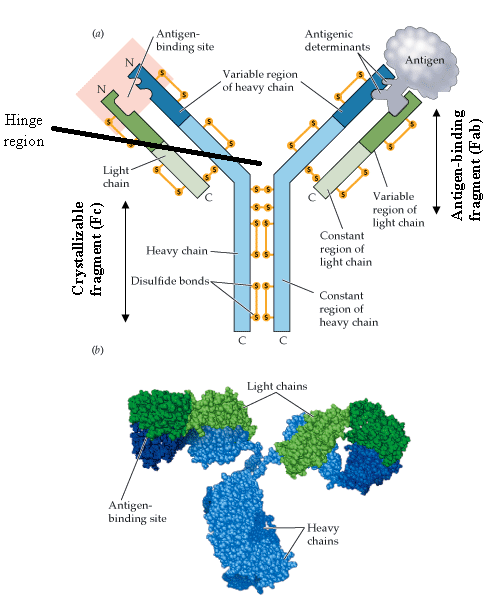
The main structure of the immunoglobulin or antibody is a monomer that resembles a Y-shaped-structure with two arms and a tail or stalk region (Figure 1). An immunoglobulin (Ig) or antibody is made up of four polypeptide chains that comprise of two identical heavy chains (H) and two identical light chains (L) that are covalently linked by disulphide bonds (Figure 1).
Each of the heavy chains and light chains also have constant (C) and variable (V) regions which varies amongst the two chains; and the polypeptide chain of Ig have amino terminal and carboxyl terminal ends which form part of the V and C regions respectively. For example, an L chain usually consists of one variable (VL) domain and one constant (CL) domain. However, the H chain of some antibodies may consist of one variable (VH) domain and more than one constant (CH) domains.
Structurally, antibodies have two main regions or fragments which are the antigen-binding fragment (Fab) and the crystallizable fragment (Fc). The Fab region is the fragment or region of the immunoglobulin that specifically binds antigens at their epitopes or antigenic determinant sites while the Fc region is the fragment that activates complements and phagocytosis. The Fc region is the site at which the immunoglobulin molecule binds to a cell, and this region lacks the affinity or ability to bind antigens.
It is noteworthy that the Fab fragments have both constant and variable regions while the Fc fragment has only the constant region. Each H chain and L chain in an antibody consist of an amino-terminal variable (V) region that is made up of several amino-acids (ranging between 100-110 amino acids); and these amino acid sequence that line the H and L chains of Ig are largely responsible for the specificity of the antigen binding site of the antibody.
The amino terminal region of the H and L chain varies greatly among antibodies. The hinge region separates the two antigen-binding sites; and it is the region of the heavy chains between the constant domains held together by disulphide bonds. The hinge region is flexible in IgA, IgD and IgG but inflexible in IgM and IgE.
REFERENCES
Abbas A.K, Lichtman A.H and Pillai S (2010). Cellular and Molecular Immunology. Sixth edition. Saunders Elsevier Inc, USA.
Actor J (2014). Introductory Immunology. First edition. Academic Press, USA.
Alberts B, Bray D, Johnson A, Lewis J, Raff M, Roberts K and Walter P (1998). Essential Cell Biology: An Introduction to the Molecular Biology of the Cell. Third edition. Garland Publishing Inc., New York.
Bach F and Sachs D (1987). Transplantation immunology. N. Engl. J. Med. 317(8):402-409.
Barrett J.T (1998). Microbiology and Immunology Concepts. Philadelphia, PA: Lippincott-Raven Publishers. USA.
Jaypal V (2007). Fundamentals of Medical Immunology. First edition. Jaypee Brothers Medical Publishers (P) Ltd, New Delhi, India.
John T.J and Samuel R (2000). Herd Immunity and Herd Effect: New Insights and Definitions. European Journal of Epidemiology, 16:601-606.
Levinson W (2010). Review of Medical Microbiology and Immunology. Twelfth edition. The McGraw-Hill Companies, USA.
Roitt I, Brostoff J and Male D (2001). Immunology. Sixth edition. Harcourt Publishers Limited, Spain.
Zon LI (1995). Developmental biology of hematopoiesis. Blood, 86(8): 2876–91.
Discover more from Microbiology Class
Subscribe to get the latest posts sent to your email.