Urine culture is performed in order to specifically identify organisms that may be causing a urinary tract infection (UTI). Urine in the bladder is often sterile as it is free from microorganisms. It becomes inundated with microorganisms (especially those of the normal micro-flora) when it leaves the bladder. UTI is very common in patients that are under catheterization and those having a poor personal hygiene.
The source of urine sample for urine culture analysis can be mid-stream urine (MSU) or clean-catch urine (CCU) from a patient using a catheter (i.e. in critically ill patients who cannot naturally pass out urine). Patients without a catheter are given sterile sample containers to collect their urine samples for bacteriological and/or microscopical analysis in the microbiology laboratory.
AIM: To isolate and identify pathogenic bacteria from urine specimens of patient’s as an aid in the diagnosis of urinary tract infections.
MATERIAL/APPARATUS: Mid – stream urine (MSU) specimen, inoculating loop, Bunsen burner, incubator, cystein lactose – electrolyte deficient (CLED) agar, blood agar (BA), grease pencil. CLED is a universal culture medium used for the isolation of uropathogens from urine samples. Other culture media such as BA and MacConkey agar are also used during urine culture.
METHOD/APPARATUS FOR URINE CULTURE
- Label the relevant agar plates (e.g. CLED and BA) with the patient’s name and laboratory number using a grease pencil.
- Mix the urine specimen by rotating the container.
- Using a sterilized inoculating loop, collect a loopful of the specimen and inoculate it on the culture media plates (e.g. CLED and BA).
- Incubate both plates in the incubator at 37oC overnight or for 18-24 hours.
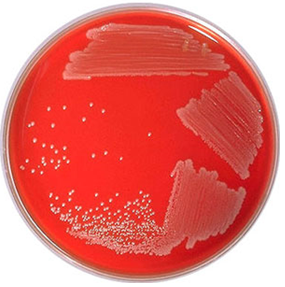
- Examine the culture media plates for significant bacterial colonies. Some common uropathogens include Staphylococcus aureus (Figure 1) and Escherichia coli (Figure 2).
- Subculture the isolated organisms onto freshly prepared culture media plates (e.g. CLED and BA) for the isolation of pure cultures.
- Perform biochemical testing (including citrate test, urease and indole test) and Gram staining as an aid in the identification of the bacterial pathogen.
- Perform antimicrobial susceptibility testing only when a pathogen has been isolated.
NOTE: It is not necessary to culture urine specimens that are microscopically and biochemically normal. Culture is only required when the urine specimen contains bacteria (as indicated by Gram smear), and the presence of pus cells, casts, nitrite, or protein in the urine sample.
REPORTING OF THE RESULT: Report bacterial numbers on culture media plate by counting the approximate number of colonies, and then estimating the number of bacteria by colony forming unit per ml (CFU/ml). For bacterial counts, the following may apply:
- Bacterial counts less than 104/ml = Not significant.
- Bacterial counts between 104 – 105/ml = Doubtful significant.
- Bacterial numbers more than 105/ml = Significant bacteriuria.
A detection threshold of 105 CFU/ml of bacterial colonies resulting from a urine culture plate is significant of a bacteriuria.
Look particularly for some common uropathogens including E. coli, Klebsiella, Pseudomonas aeruginosa and Staphylococcus aureus on the culture media plate such as CLED plate, BA plates, cetrimide selective agar and MacConkey plates.
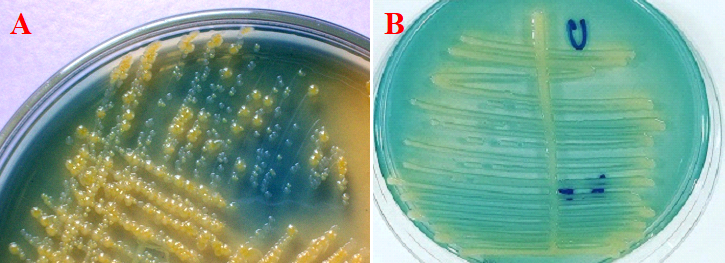
Cetrimide selective agar is used for the selective isolation of Pseudomonas aeruginosa isolates from clinical samples. P. aeruginosa produces greenish colonies on cetrimide selective agar due to the formation of pyocyanin and pyoverdin; and the production of this greenish colony is aided by the addition of glycerol to the culture medium during its preparation (Figure 3).
S. aureus cause haemolysis on BA, and this feature can be used to distinguish it from other bacteria. And they also produce small circular distinct colonies on BA plates. Other pathogenic microorganisms found in urine include Candida species, Streptococcus pneumoniae, Proteus mirabilis, Enterococcus species and Listeria monocytogenes. These organisms can be identified and/or isolated from the urine specimen by expanding the number of culture media plates used for the urine culture process.
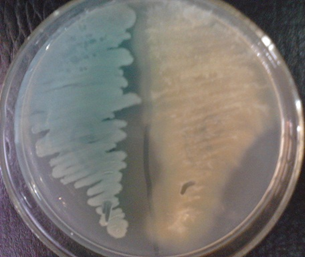
Blood agar (BA) is used to isolate fastidious organisms e.g. Staphylococcus and Streptococcus that require additional nutrient or growth factor like blood to grow.
Cysteine lactose electrolyte deficient (CLED) medium is a non-inhibitory differential medium which is specially used to isolate urinary pathogens from urine specimens. It allows the growth of both Gram positive and Gram negative pathogens. Lactose fermenting organisms appear yellow on CLED because the agar is bromothymol blue.
CLED is electrolyte – deficient, and this prevents the growth of swarming organisms like Proteus species. The swarming nature of Proteus mirabilis colonies can be identified on blood agar plates in the laboratory.
References
Basic laboratory procedures in clinical bacteriology. World Health Organization (WHO), 1991. Available from WHO publications, 1211 Geneva, 27-Switzerland.
Beers M.H., Porter R.S., Jones T.V., Kaplan J.L and Berkwits M (2006). The Merck Manual of Diagnosis and Therapy. Eighteenth edition. Merck & Co., Inc, USA.
Biosafety in Microbiological and Biomedical Laboratories. 5th edition. U.S Department of Health and Human Services. Public Health Service. Center for Disease Control and Prevention. National Institute of Health. HHS Publication No. (CDC) 21-1112.2009.
Cheesbrough M (2010). District Laboratory Practice in Tropical Countries. Part I. 2nd edition. Cambridge University Press, UK.
Cheesbrough M (2010). District Laboratory Practice in Tropical Countries. Part 2. 2nd edition. Cambridge University Press, UK.
Collins C.H, Lyne P.M, Grange J.M and Falkinham J.O (2004). Collins and Lyne’s Microbiological Methods. Eight edition. Arnold publishers, New York, USA.
Disinfection and Sterilization. (1993). Laboratory Biosafety Manual (2nd ed., pp. 60-70). Geneva: WHO.
Garcia L.S (2010). Clinical Microbiology Procedures Handbook. Third edition. American Society of Microbiology Press, USA.
Garcia L.S (2014). Clinical Laboratory Management. First edition. American Society of Microbiology Press, USA.
Fleming, D. O., Richardson, J. H., Tulis, J. I. and Vesley, D. (eds) (1995). Laboratory Safety: Principles and practice. Washington DC: ASM press.
Dubey, R. C. and Maheshwari, D. K. (2004). Practical Microbiology. S.Chand and Company LTD, New Delhi, India.
Gillespie S.H and Bamford K.B (2012). Medical Microbiology and Infection at a glance. 4th edition. Wiley-Blackwell Publishers, UK.
Discover more from Microbiology Class
Subscribe to get the latest posts sent to your email.