Rickettsia prowazekii is an obligate intracellular parasite that causes a variety of rickettsial related diseases in humans. It is the main causative agent of epidemic typhus in man. Epidemic typhus is a disease that occurs worldwide, and it is mainly transmitted to humans via the bite of lice. Obligate intracellular parasites are organisms that only replicate inside a living host cell. Such organisms termed as obligate intracellular parasites do not survive long outside their host cells. Rickettsia species are Gram-negative, pleomorphic, aerobic and non-flagellated organisms that can only reproduce in living host cells.
They exist as short rods, cocci or coccobacilli, and measure between 0.3-1 µm. R. prowazekii is found in the genus Rickettsiaceae; and species in this genus are naturally occurring parasites that can be found in the gut of some arthropods or insects (e.g. ticks and mites), wild and domestic animals and in rodents. Dogs and rodents also act as reservoirs for Rickettsia species. Though they exhibit viral-like properties and cannot survive as free-living microorganisms, Rickettsia species contain both DNA and RNA, have peptidoglycan cell walls and they can reproduce by binary fission.
Thus, Rickettsia species are categorized as bacteria than viruses even though they can only replicate only in living cells (a property unique to viruses). Their requirement for certain host cell components or material for growth such as adenosine triphosphate (ATP) and some nucleotide cofactors are some of the features that Rickettsia species share with viruses. Rickettsia species like viruses discontinue their metabolic activity once outside a living host cell. Rickettsia species are slow growing, and they can only be cultured or isolated in tissue cultures of living eukaryotic cells and in vivo in certain laboratory animals (e.g. rat).
Outside a living cell, the membranes of Rickettsia species becomes permeable and important growth nutrients such as proteins and nucleic acids are lost to the exterior via their porous cell surfaces. This makes it impossible for the organism (which are generally strict obligate intracellular parasites) to be propagated extracellularly because they cannot obtain growth nutrients this way. Species of Rickettsiaceae are mainly transmitted by arthropods or insects (e.g. body lice, rat fleas and ticks), and they cause a number of human infections generally regarded as rickettsioses e.g. typhus fever, Q fever and Rocky Mountain Spotted Fever (RMSF).
PATHOGENESIS OF RICKETTSIA PROWAZEKII INFECTION
Human infections or diseases caused by Rickettsia species occur via the bite of an infected insect or arthropod that harbours and transmits the parasite during a blood meal. Arthropods that transmit Rickettsia species to humans pick up the parasite when they feed on the blood of infected animals (e.g. cow, goat and sheep), and the parasite is transmitted directly to humans during blood meal or indirectly through scratching by contaminating the bite with its feaces (that contains numerous amount of Rickettsia)during the blood meal. These animals also play vital roles in the life cycle of the insect vectors that transmit Rickettsia parasites to humans.
In effect, virtually all rickettsial diseases are zoonoses (i.e. animal infections transmitted to humans) since all the parasites have animal reservoirs excluding R. typhus that only has a mammalian or human reservoir and the causative agent of Q fever that is exclusively transmitted via aerosols. Once inside the body, Rickettsia parasites circulate widely in the blood stream, and they primarily infect the vascular endothelial cells of the body resulting in vasculitis and thrombosis.
Vasculitis is the inflammation of the blood vessels while thrombosis is the formation of blood clots inside blood vessels (arteries and veins) in such a way that the free flow of blood in the body’s circulatory system is prevented.Rupture and necrosis of blood vessels occur, leading to the release of rickettsial parasitesthat become systemic. The incubation period of the parasite and/or disease is about 3 weeks, and this phase is marked by the multiplication of Rickettsia in the blood vessels and other cells of the infected human host.
R. prowazekii and other Rickettsia species produce cell degrading substances such as phospholipases and other virulent factors which allow them to degrade host cell components thus allowing them to penetrate the cytoplasm where they produce a variety of cytopathic effects similar to that caused by viral organisms. The reproduction or multiplication of Rickettsia species within their host cells is capable of causing the death of the cell. Some Rickettsia species (e.g. R. rickettsii)can also live in the nucleus of the host cell they invade as well as in the cytoplasm.
R. prowazekii is transmitted to humans via the bite of body louse (in particular, the Pediculus louse species), and it is the main causative agent of louse-borne epidemic typhus. Several typhus infections caused by Rickettsia species exist and this includes scrub typhus (caused by R. tsutsugamushi) and flea-borne epidemic typhus (caused by R. typhi) amongst other forms of rickettsial typhus infections. Louse-borne epidemic typhus can be transmitted from one person to another by the body louse. The louse ingest the parasite after taking blood meal from an individual, and the ingested Rickettsia species migrates to the insect’s gut where it multiplies, and then released via the feaces in large numbers.
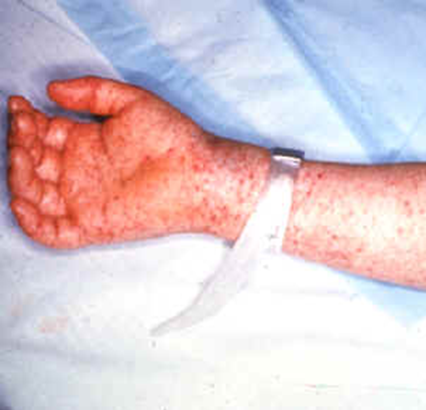
When the louse feeds on another person, it releases Rickettsia in its feaces and the bitten human host assists the parasite to enter its body as the individual scratches the bite site. The irritation caused by the louse bite causes the bitten individual to scratch the site, and this act inoculates the parasite into the bite wound. Rocky Mountain Spotted Fever (RMSF) is another severe form of disease caused by Rickettsia species. RMSF is an acute febrile infection caused by R. rickettsii, and it is amongst the most virulent species of Rickettsiaceae (R. prowazekii inclusive). It is a serious and potentially life-threatening infectious disease that affects humanity even till date, and it is clinically manifested as a characteristic rash that appears on the entire body of the affected individual excluding the face, palms and feet of the affected individual (Figure 1).
Q fever is caused by Coxiella burnetii (an obligate intracellular parasite that is related to species of Rickettsiaceae), and the parasite affects the human lungs producing a pneumonia-like type of infection. Q means “query” because the causative agent of the disease was largely unknown the first time the infection was described. C. burnetii survives in the air because it is resistant to drying, and human infection with the parasite usually occurs via the inhalation of aerosols or dust particles contaminated with C. burnetii from dried animal feaces or urine. Infected or contaminated food products (e.g. milk) also serve as route via which the pathogen can be transmitted to humans.
The causative agent of Q fever is different from Rickettsia species in that the former form resistant endospores that enable it to survive outside the body of a living host but Rickettsia cannot survive outside a living host cell. C. burnetii is not transmitted directly to humans via insect bite but it can be transmitted to animals (e.g. goat, sheep and cow) via insect bite, and these animals shed large amount of the parasite in their urine, feaces and other body fluids. The inhalation of aerosols or dust particles contaminated with the feaces of any of these animals by man can cause Q fever in the individual. Slaughter houses and animal farms are examples of places where human infection with C. burnetii can easily occur.
Q fever, RMSF and epidemic typhus are all characterized by similar clinical signs and symptoms including fever, vomiting, abdominal pain, myalgia, body weakness, nausea, headache and rashes all over the body (for RMSF) but there is no sign of skin rash in C. burnetii infection in humans. Enlargement of the spleen and liver rarely occurs in some severe infections caused by Rickettsia species. Mortality due to rickettsial infections can ensue in very severe cases of the disease, and this is usually attributed to systemic infections that affect some vital organs of the body such as the heart (causing endocarditis), brain (causing encephalitis) and lungs (causing pneumonia).
LABORATORY DIAGNOSIS OF RICKETTSIA PROWAZEKII INFECTION
For rickettsial infections, laboratory diagnosis is primarily dependent on the detection and identification of the invading rickettsial cell, and this is usually made by in vitro cell/tissue culture techniques and in vivo cultivation in experimental animals such rats and mice. The difficulty and hazardous nature associated with the in vitro cultivation of Rickettsia species in cell cultures is the reason why most rickettsial investigations are only undertaken in reference laboratories. The laboratory diagnosis of Q fever is usually made serologically using a variety of complement fixation test (CFT) and immunoflourescence test (IT).
Latex agglutination tests, PCR-based nucleic acid assays (to detect rickettsial specific DNA sequence) and a number of antibody-based tests (which detect specific surface antigens on rickettsial cells) are now currently employed for the rapid detection of rickettsial infections. Generally, serodiagnosis (i.e. serological analysis) is the most widely available and frequently used techniques for the confirmation of rickettsial diseases; and these methods are often performed than the cell culture and in vivo experimental techniques in laboratory animals.
IMMUNITY TO RICKETTSIA PROWAZEKII INFECTION
Immunity to rickettsial infection is marked by an early development of innate immune response with the activation of natural killer (NK) cells and the production of cytokines (e.g. interferons) as well as the development of acquired immunity (which is manifested as clonal expansion of B cells for antibody production and lymphocyte production respectively). Since rickettsial cells are strict obligate intracellular parasites, the cell mediated immune response (usually involving the CD4 and CD8 T lymphocytes) plays vital role in stopping parasite multiplication in vivo. Despite the protective function of antibody production and the vital role played by the cell mediated immune system cells, recrudescent typhus – which is a less severe relapsing fever of typhus can occur in the later years of previously infected individuals.
TREATMENT OF RICKETTSIA PROWAZEKII INFECTION
Rickettsial infections are best treated with the daily administration of antibacterial agents such as chloramphenicol and tetracyclines or doxycycline which are very effective against the pathogen once treatment is started on time. Sulphonamides are contraindications for the treatment of rickettsial infection and therefore should not be used because the agent encourages the multiplication of the parasite in vivo. Treatment should be started immediately and not delayed once rickettsial infection has been clinically suspected, and this should be done prior to the confirmation of laboratory test results.
Antibiotic therapy is very much effective during the first week of infection than in later weeks, and thus should be promptly administered upon the suspicion of an infection. The use of tetracycline in pregnant women should not be anticipated but instead be replaced with doxycycline due to some risk the former portend in the unborn child. The host immune system is responsible for the total clearing of the parasite from the system since the antibiotic therapy only suppresses the growth of the rickettsial cells.
PREVENTION AND CONTROL
Most rickettsial infections (e.g. epidemic typhus and scrub typhus) are clinically important and prevalent in developing countries. Those at high risk of infection with any of the Rickettsia species include those whose occupation constantly exposes them to animal feaces, urine and other body fluids; and this includes people working in abattoirs, shepherdess and shepherd, farm workers and those in engaged in livestock breeding.
Since the majority of Rickettsia species (excluding C. burnetii which is transmitted via aerosols) are mainly maintained in certain types of insects or arthropods (e.g. tick, flea, lice and mite) that feeds on human blood (after taking blood meal from their animal reservoirs) and transmits the parasite in the process, it is critical to prevent insect bite as much as possible via vector control and people should also endeavour to maintain good personal hygiene. People should also avoid tick infested areas and other places where the parasite vectors are known to thrive.
Most cases of rickettsial infections have been reported in military camps and in refugee camps where sanitation and personal hygiene is extremely poor. Delousing (i.e. removal of louse from the body) in louse-infested individuals and tick removal (deticking) from the body of tick infected persons is critical in the prevention of typhus infection. The use of chemical agents to control the insect vectors of Rickettsia species in vector endemic areas will also help to control the transmission of the disease.
OTHER SPECIES OF RICKETTSIA
- R. rickettsii: R. rickettsii is a Gram-negative, intracellular, coccobacillus and an important member of the genus Rickettsia. It is the causative agent of Rocky Mountain spotted fever (RMSF) that is characterized by spotted rash appearance throughout the body of infected persons.
- R. africae: R. africae is the species of Rickettsia that cause African tick-bite fever, a highly prevalent infection in Africa. The organism is a tick-borne pathogen that affects travelers to Sub-Saharan Africa. Human infection with R. africae usually occurs through contact with ticks that parasitize cattle or wild animals, especially the amblyomma ticks.
- R. typhi (formerly R. mooseri): R. typhi is the etiologic agent of murine or endemic typhus. The organism is transmitted primarily by the rat flea, Xenopsylla cheopis. However, lice and mites are other potential vectors of the organism. R. typhi is transmitted from infected rats to humans chiefly by rat fleas.
- R. tsutsugamushi (now called Orientia tsutsugamushi): R. tsutsugamushi is the causative agent of scrub typhus, a rickettsial disease that is common in Asia and Australia. The vector and reservoir of the organism is mainly the trombiculid mites. R. tsutsugamushi is a highly virulent organism.
- R. conorii: R. conorii is the causative agent of several rickettsial infections including but not limited to Boutonneuse fever, Mediterranean spotted fever, Israeli tick typhus, Astrakhan spotted fever, Kenya tick typhus, and Indian tick typhus depending on the geographical location of the infection or the causative agent of the disease. The most prevailing vector of R. conorii is the brown dog tick known as Rhipicephalus sanguineus. R. conorii isthe most geographically dispersed or widespread species of the genus Rickettsia.
- R. japonica: R. japonica is the causative agent of Japanese spotted fever – which is common in Asia (particularly Japan). Japanese spotted fever is geographically distributed in Japan.
- R. felis: R. felis is the causative agent of flea-borne spotted fever in cats. And R. felis can also infect humans to cause similar infection. R. felis is mainly harboured by fleas; and the organism is most prevalent in South and Northern America.
- R. slovaca: R. slovaca is a pathogenic, tick-borne, spotted fever group (SFG) of the rickettsial familay that was initially isolated in from a Dermacentor marginatus tick in Slovakia. But the disease has spread across Europe. R. slovaca causes Tick-borne lymphadenopathy (TIBOLA) and Dermacentor-borne necrosis and lymphadenopathy (DEBONEL).
- Rickettsia aeschlimannii: Rickettsia aeschlimannii is the causative agent of Rickettsiosis, which is geographically restricted to the Mediterranean and Africa.
References
Brooks G.F., Butel J.S and Morse S.A (2004). Medical Microbiology, 23rd edition. McGraw Hill Publishers. USA. Pp. 248-260.
Madigan M.T., Martinko J.M., Dunlap P.V and Clark D.P (2009). Brock Biology of microorganisms. 12th edition. Pearson Benjamin Cummings Publishers. USA. Pp.795-796.
Prescott L.M., Harley J.P and Klein D.A (2005). Microbiology. 6th ed. McGraw Hill Publishers, USA. Pp. 296-299.
Ryan K, Ray C.G, Ahmed N, Drew W.L and Plorde J (2010). Sherris Medical Microbiology. Fifth edition. McGraw-Hill Publishers, USA.
Singleton P and Sainsbury D (1995). Dictionary of microbiology and molecular biology, 3rd ed. New York: John Wiley and Sons.
Talaro, Kathleen P (2005). Foundations in Microbiology. 5th edition. McGraw-Hill Companies Inc., New York, USA.
Discover more from Microbiology Class
Subscribe to get the latest posts sent to your email.