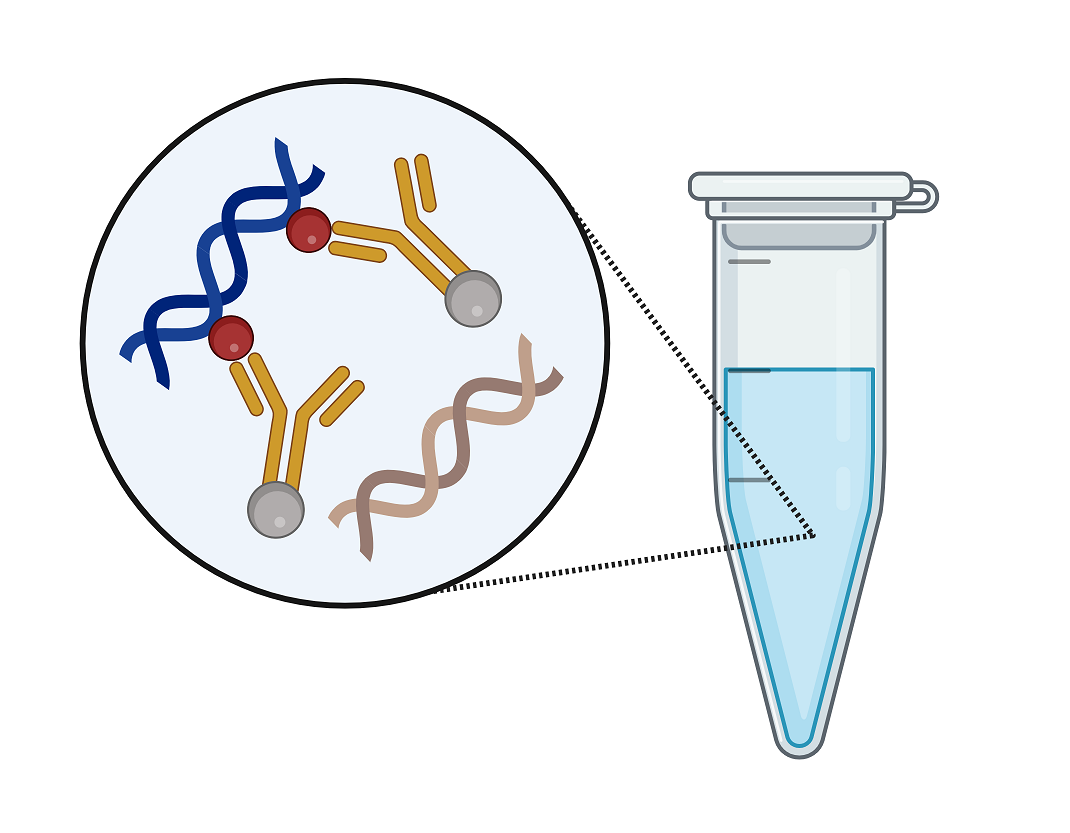
TaqMan® probe signal production
Whether an MGB or non-MGB probe is chosen, both follow the same pattern for signal production. In the early PCR cycles, only the low, quenched reporter signal is detected. This early signal, automatically subtracted to zero in the real-time PCR software, is termed “baseline”. If the sample contains a target, eventually enough of the cleaved probe will accumulate to allow amplification signal to emerge from the baseline. The point at which amplification signal becomes visible is inversely related to the initial target
quantity.
SYBR® Green dye
SYBR® Green I dye is a fluorescent DNA-binding dye that binds to the minor groove of any double-stranded DNA. Excitation of DNA-bound SYBR® Green dye produces a much stronger fluorescent signal compared to unbound dye. A SYBR® Green dye–based assay typically consists of two PCR primers. Under ideal conditions, a SYBR® Green assay follows an amplification pattern similar to that of a TaqMan® probe–based assay. In the early PCR cycles, a horizontal baseline is observed. If the target was present in the sample, sufficient accumulated PCR product will be produced at some point so that amplification signal
becomes visible.
SYBR® Green assay specificity
Assay specificity testing is important for all assays, but especially for those most vulnerable to specificity problems. SYBR® Green assays do not benefit from the specificity of a TaqMan® probe, making them more vulnerable to specificity problems. SYBR® Green dye will bind to any amplified product, target or non-target, and all such signals are summed, producing a single amplification plot. SYBR® Green amplification plot shape cannot be used to assess specificity. Plots usually have the same appearance, whether the amplification consists of target, non-target, or a mixture. The fact that a SYBR® Green assay produced an
amplification should not be automatically taken to mean the majority of any of the signal is derived from target. Since amplification of non-target can vary from sample to sample, at least one type of specificity assessment should be performed for every SYBR® Green reaction. Most commonly, this ongoing assessment is the dissociation analysis.
SYBR® Green dye dissociation
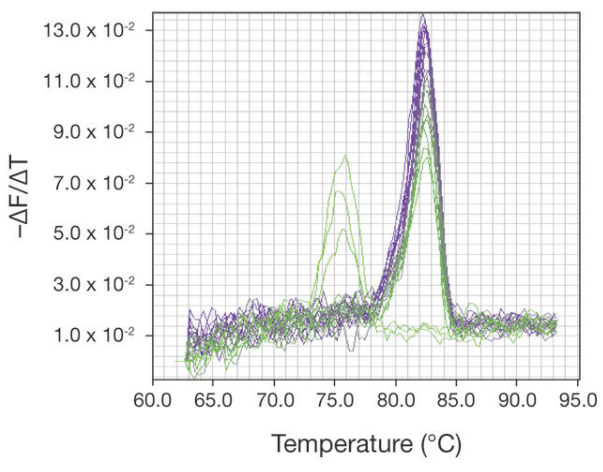
SYBR® Green dissociation is the gradual melting of the PCR products after PCR when using SYBR® Green–based detection. Dissociation is an attractive choice for specificity assessment because it does not add cost to the experiment and can be done right in the PCR reaction vessel. However, dissociation does add more time to the thermal protocol, requires additional analysis time, is somewhat subjective, and has limited resolution. The concept of SYBR® Green dissociation is that if the target is one defined genetic sequence, it should have one specific melting temperature (Tm), which is used to help identify the target in samples. Some non-target products will have Tm values significantly different from that of the target, allowing detection of those non-target amplifications.
The dissociation protocol is added after the final PCR cycle. Following the melt process, the real-time PCR software will plot the data as the negative first derivative, which transforms the melt profile into a peak. Accurate identification of the target peak depends on amplification of pure target. Many samples such as cellular RNA and genomic DNA exhibit high genetic complexity,
creating opportunities for non-target amplification that may suppress the amplification of the target or, in some cases, alter the shape of the melt peak. By starting with pure target, the researcher will be able to associate a peak Tm and shape with a particular target after amplification. Only one peak should be observed. The presumptive target peak should be narrow, symmetrical, and devoid of other anomalies, such as shoulders, humps, or splits. These anomalies are strong indications that multiple products
of similar Tm values were produced, casting strong doubts about the specificity of those reactions.
Wells with dissociation anomalies should be omitted from further analysis. SYBR® Green dissociation is low resolution and may not differentiate between target and non-target with similar Tm values (e.g., homologs). Therefore, one, narrow symmetric peak should not be assumed to be the target, nor one product, without additional supporting information. Dissociation data should be evaluated for each well where amplification was observed. If the sample contains a peak that does not correspond to the pure target peak, the conclusion is that target was not detected in that reaction.
If the sample contains a peak that appears to match the Tm and shape of the pure target peak, target may have amplified in that reaction. Dissociation data in isolation cannot be taken as definitive, but when combined with other information, such as data from target-negative samples, sequencing, or gels, can provide more confidence in specificity.
Real-time PCR instrumentation
Many different models of real-time PCR instruments are available. Each model must have an excitation source, which excites the fluorescent dyes, and a detector to detect the fluorescent emissions. In addition, each model must have a thermal cycler. The thermal block may be either fixed, as in the StepOnePlus™ system or user interchangeable, as in the ViiA™ 7 system, the QuantStudio® 6 and 7 Flex systems, and QuantStudio® 12K Flex system. Blocks are available to accept a variety of PCR reaction
vessels: 48-well plates, 96-well plates, 384-well plates, 384-microwell cards, 3,072–through-hole plates, etc. All real-time PCR instruments also come with software for data collection and analysis.
Dye differentiation
Most real-time PCR reactions contain multiple dyes, including one or more reporter dyes, in some cases a quencher dye, and, very often, a passive reference dye. Multiple dyes in the same well can be measured independently, either through optimized combinations of excitation and emission filters or through a process called multicomponenting. Multicomponenting is a mathematical method to measure dye intensity for each dye in the reaction. Multicomponenting offers the benefits of easy correction for dye designation errors, refreshing optical performance to factory standard without hardware adjustment, and provides a source of troubleshooting information.
Source
www.lifetechnologies.com
Discover more from Microbiology Class
Subscribe to get the latest posts sent to your email.