The phrase liposome is derived from two Greek words: Lipos which means “fat” and Soma which means “body”. Liposomes (also known as closed bilayer phospholipid systems) are artificial spherical vesicles that possess several or at least one lipid bilayer for the incorporation of an active agent such as a drug. They are often used as vehicles for the administration of nutrients and pharmaceutical agents.
Liposomes are usually prepared through the process of sonication – in which the lipid bilayers and/or biological membranes of living systems are disrupted in a controlled and precise manner. The use of liposomes as drug-delivery systems was first proposed in the early 1960s; and ever since then liposomes have been employed in several medical and/or pharmaceutical applications especially in the area of target drug delivery.
Liposomes were first discovered and described by the British haematologist Dr Alec D. Bangham in 1961 at the Babraham Institute, in Cambridge when Bangham and colleagues were testing their institute’s new electron microscope while working on phospholipids. Their discovery helped them to accurately describe the true structure of the phospholipid bilayer of living systems.
One of the most important reasons for incorporating or encapsulating drugs into liposomes prior to their administration in vivo is for no other reason than to protect the active constituent of the drug from possible metabolic degradation in the system of the host. Also, drugs are encapsulated into liposomes in order to restrict their mode of action and ensure that they release their content only at the target site of action; and this is usually applicable in the administration of drugs into the bloodstream of a host.
Apart from drugs and nutrients, enzymes can also be encapsulated into liposomes through the process of immobilization. Enzyme immobilization is the attachment of an enzyme to a solid surface or structure in order to prevent their possible degradation prior to their use or during use. Liposomes can be filled with drugs, and used to deliver medicines for different infectious and non-infectious diseases including but not limited to tuberculosis and cancer. The biological membranes of living organisms are usually made of phospholipids, which are molecules that have a head group and a tail group (Figure 1). Phospholipids are usually found in nature in stable membranes composed of two layers. This stable biological membrane of two layers is generally known as a bilayer.
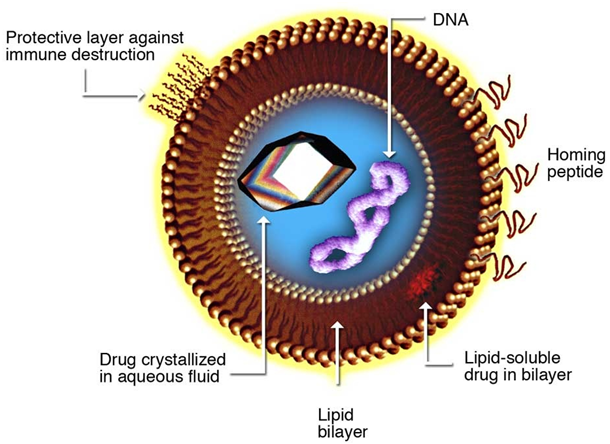
A typical phospholipid structure possesses two important parts which are the hydrophilic head and the hydrophobic tail as shown in Figure 2. The hydrophilic head is water-loving while the hydrophobic tail of a phospholipid molecule is water-hating. This implies that the head of a phospholipid molecule is attracted to water while the tail is repelled by water. In the presence of water, the hydrophilic heads of a phospholipid are attracted to water, and thus the heads line up to form a surface that faces the water. However, the hydrophobic tails which are naturally repelled by water are lined up to form a surface away from the water.
In a living cell, one layer of the hydrophilic heads faces outside of the cell because it is attracted to the water in the cell’s environment while another layer of the hydrophilic heads faces inside the cell because it is attracted by the water within the cell. The hydrocarbon tails of one layer face the hydrocarbon tails of the other layer, and the combined structure forms a bilayer. Liposomes represent an advanced technology especially in the field of nanomedicine to deliver active biological molecules such as drugs to the site of action in vivo; and liposomes are promising drug-delivery systems currently being employed in pharmaceutical companies for the production of drugs.
A recent technology in the use of liposomes to deliver active agents in vivo is the Liposomal Encapsulation Technology (LET) that makes it possible to deliver essential nutrients and drugs directly into the bloodstream of an individual. Liposomes can be created from cholesterol and natural non- toxic phospholipids. When the membrane phospholipids of a living system are disrupted, they can reassemble themselves into tiny spheres, smaller than a normal cell, either as bilayers or monolayers.
The bilayer structures formed are known as the liposomes while he monolayer structures formed are called micelles. Generally, liposomes act as drug carriers, and in this manner they help in the delivery of therapeutic agents to their target sites and at potent therapeutic concentrations. Several drug formulations (e.g. Amphotericin B) that make use of liposomal drug encapsulation are in clinical use around the world. Irrespective of their invaluable medical/pharmaceutical applications, the use of liposomes in drug delivery systems is not without some flaws as shown in Table 1.
Examples of drugs and/or pharmaceutical formulations that are delivered and used as therapeutic agents with the help of liposomal carriers include:
- Amphotericin B,
- Insulin,
- Vaccines,
- Corticosteroids,
- Anesthetics,
- Antibiotics,
- Immunomodulators,
- Porphyrins, and
- Some peptides.
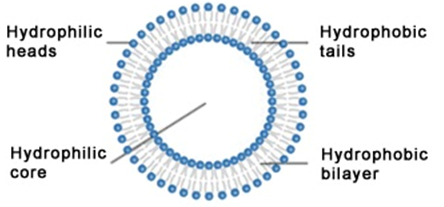
Table 1. Advantages and disadvantages of liposomes
| Advantages | Disadvantages |
| Liposomes increased efficacy and therapeutic index of drugs | Liposomes have low solubility |
| Liposomes increased drug stability via encapsulation | Liposomes have short half-life |
| They are non-toxic, flexible, biocompatible, completely biodegradable, and non-immunogenic for systemic and non- systemic administration | Sometimes phospholipids undergoes oxidation and hydrolysis-like reaction |
| Liposomes reduces the toxicity of the encapsulated drug | There is possible leakage and fusion of encapsulated drug/molecules |
| Liposomes help reduce the exposure of sensitive tissues to toxic drugs | The production cost of liposomes is high |
| Liposomes have site avoidance effect | They have fewer stability in vivo |
CHARACTERISTICS OF LIPOSOMES USED FOR DRUG DELIVERY SYSTEMS
To be effective for targeted drug delivery, a liposomal material must meet certain criteria as follows:
- The liposomes exterior must be hydrophilic. Therefore encapsulating hydrophobic drugs with liposomes increases water solubility.
- Liposomes must protect the drugs from possible degradation in bloodstream of the host. This increases the efficacy of the encapsulated drug and thus lowers drug toxicity to the host’s organs.
- Drugs encapsulated in the liposomes can be delayed release. By the variation of lipid components and particle sizes, pharmaceutical kinetics can be altered – thereby increasing concentration in the blood and distribution.
- Liposomes must carry the encapsulated drug in the systemic circulation to the infected area and release the contents for targeted delivery. This helps to decrease adverse effects of the drug in the host.
- Liposomes can also be used for gene delivery to host cells thereby enhancing efficacy.
- Liposomes must increase drugs permeation while extending the retention time of the drugs locally. And it must lower possible irritation and inflammation to the host’s skin as well as decrease any systemic toxicity that may ensue.
- Similar phospholid bilayer structures are observed between liposomes and cell membranes, thus increasing biocompatability and biodegradability while decreasing drug toxicity.
References
Bains W (1998). Biotechnology: From A to Z. 2nd ed. Oxford University Press, New York, USA.
Bourgaize D, Jewell T.R and Buiser R.G (1999). Biotechnology: Demystifying the Concepts. Pearson Education, San Francisco, CA.
Brian Robert Shmaefsky (2006). Biotechnology 101. Greenwood Publishing Group, Inc, USA. Pp. 1-273.
Bushell M.E (1998). Application of the principles of industrial microbiology to biotechnology (ed. Wiseman, A.) Chapman and Hall, New York. Pp. 5–43.
Byong H. Lee (2015). Fundamentals of Food Biotechnology. Second edition. Wiley-Blackwell, New Jersey, United States.
Chrispeels M.J and Sadava D.E (2002). Plants, Genes, and Crop Biotechnology. 2nd edition. Jones and Bartlett Publishers, Sudbury, MA.
Clark D.P and Pazdernik N (2010). Biotechnology. First edition. Elsevier Science and Technology Books, Amsterdam, Netherlands.
Das H.K (2010). Textbook of Biotechnology. Fourth edition. Wiley edition. Wiley India Pvt, Ltd, New Delhi, India.
Dictionary of Microbiology and Molecular Biology, 3rd Edition. Paul Singleton and Diana Sainsbury. 2006, John Wiley & Sons Ltd. Canada.
Glick B.R and Pasternak J.J (2003). Molecular Biotechnology: Principles and Applications of Recombinant DNA. ASM Press, Washington DC, USA.
Godbey W.T (2014). An Introduction to Biotechnology. First edition. Woodhead Publishing, Cambridge, United Kingdom.
Jee C and Shagufta (2007). Environmental Biotechnology. APH Publishing Corporation, Darya Ganj, New Delhi, India.
Lee S.Y, Lee D.Y and Kim T.Y (2005). Systems biotechnology for strain improvement. TRENDS in Biotechnology, 23(7):349-356.
Discover more from Microbiology Class
Subscribe to get the latest posts sent to your email.