Cell immobilizationis the process of fixing the cells of living organisms including plants, animals, enzymes and microbial cells in a suitable matrix capable of holding them in place. The term “immobilization” is generally used to describe the technique of attaching cells to surfaces either by entrapment or by particle attachment. And the cell immobilization technique actually began with the immobilization of enzymes for various technological processes. Cell immobilization technology is aptly suited to produce extracellular enzymes. And since microorganisms are the best sources for the production of these useful enzymes, there is currently a growing interest in applying cell immobilization techniques for the continuous production of enzymes used for various medical, industrial and pharmaceutical processes.
Today, immobilization of cells have been applied in various areas including but not limited to wastewater management, pharmaceutical industries, food industries and in other fermentation processes. Whole cells, microbial cells and enzymes can be immobilized. The immobilized whole cell system is an alternative to enzyme immobilization. In enzyme immobilization, the enzyme is attached to a solid support such as calcium alginate. But in immobilized whole cell systems, the target cell is immobilized.
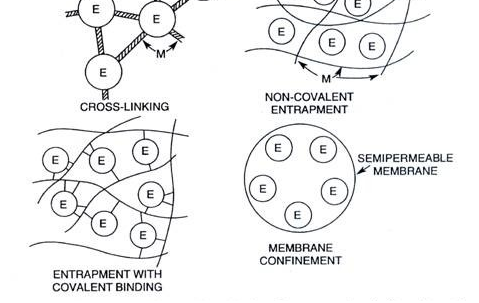
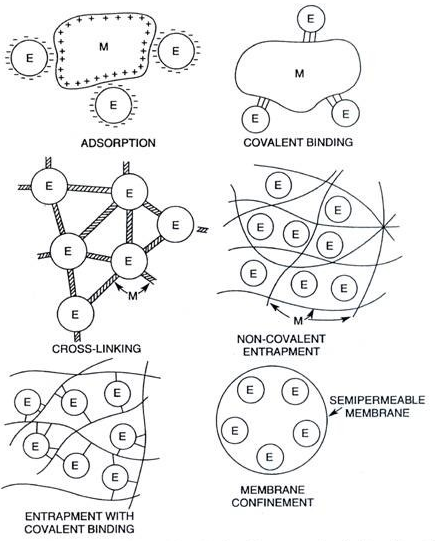
The immobilization of microbial cells in biological processes can occur either as a natural phenomenon or through artificial process. While the attached cells in natural habitat exhibit significant growth due to unlimited access to growth factors, the artificially immobilized cells are allowed restricted growth because their growth nutrients are usually limited. The immobilization of cells is mainly achieved using a high molecular hydrophilic polymeric gel such as alginate, carrageenan, and agarose; and these materials are unique in their capacity to hold cells in place. Adsorption, covalent bonding, cross linking, entrapment, and encapsulation are some of the major immobilization methods.
Adsorption is the physical binding of cells to a carrier molecule. It is the first example of cell immobilization technique and it is commonly used due to the ease to reverse the reaction. It is based on the physical interaction between the carrier molecule or surfaces and the microbial cell. Adsorption technique is relatively cheap, simple and effective to perform; and microorganisms adsorbed to proper adsorbents have the ability to get protection from unfavourable substances that may interfere with their growth. Adsorption technique also stimulate microbial metabolism and it preserves the physiological activity of the immobilized microbial cell during use.
The fixation of the microbial cell to the adsorbent is usually carried out under normal growth conditions; and adsorption technique of cell immobilization does not require additional chemicals to facilitate the process. Wood, glass ceramic and plastic materials are some of the adsorbents or supports that are used in immobilizing microbial cells using the adsorption technique.
Covalent bonding as an immobilization technique is usually based on covalent bond formation between the microbial cell and the activated inorganic support or carrier molecule in the presence of a binding agent. The binding agent in cross bonding forms a cross linking that connects both the activated inorganic support and the cell to be immobilized by this technique.
Unlike in adsorption where there is no chemical modification of the carrier molecule, there is usually a chemical modification of the surfaces of the inorganic support so that binding and/or immobilization will be more efficient. Cross linking is another immobilization technique in which microbial cells can be immobilized by cross-linking them with each other using certain chemicals or reagents with bi- or multifunctional reagents such as glutaraldehyde (Figure 1).
Entrapment is the immobilization technique in which microbial cells are entrapped in a support matrix or inside fiber materials where they are held in place (Figure 1). The entrapment of microbial cells in polymer matrices is one of the most extensively studied methods in cell immobilization techniques. Alginate, carrageenan, cellulose and its derivatives, collagen, gelatin, epoxy resin, photo cross-linkable resins, polyacrylamide, polyester, polystyrene and polyurethane are some of the polymer matrices used in entrapment immobilization technique for the immobilization of microbial cells.
Precipitation, ion exchange gelation, and polymerization are some of the network formation procedures employed in the entrapment immobilization technique. And each of these procedures is affected by several physical and/or environmental factors. The entrapment methods are usually based on the inclusion of cells within a rigid network to prevent the cells from diffusing into surrounding medium while still allowing penetration of substrate for microbial cell growth or for the growth of the entrapped cell.
The protection or entrapment provided by the entrapment immobilization technique ensures the sustenance of the viability of the microbial cell both during storage and even when in use. Entrapment immobilization technique is an irreversible immobilization process that cannot be reversed once started. Some of the demerits associated with the use of entrapment technique in the immobilization of microbial cells include limitation of diffusion of other useful materials into the matrix, possible leakage of the cells, abrasion of the support material and the high cost of the immobilization technique. Nevertheless, the entrapment immobilization provides high mechanical strength of the immobilized cell.
Encapsulation immobilization technique is similar to entrapment. Encapsulation has the advantage of high cell loading despite the diffusion limitation that is associated with it as is applicable to the entrapment technique. In encapsulation technique, the material or cell to be immobilized are restricted or confined within a membrane that is in a form of a capsule (Figure 1). Encapsulation is an irreversible immobilization technique like entrapment, and it allows for easy flow of nutrients and other substrates due to the semi-permeable nature of the membrane used for the immobilization process.
References
Bains W (1998). Biotechnology: From A to Z. 2nd ed. Oxford University Press, New York, USA.
Bourgaize D, Jewell T.R and Buiser R.G (1999). Biotechnology: Demystifying the Concepts. Pearson Education, San Francisco, CA.
Brian Robert Shmaefsky (2006). Biotechnology 101. Greenwood Publishing Group, Inc, USA. Pp. 1-273.
Bushell M.E (1998). Application of the principles of industrial microbiology to biotechnology (ed. Wiseman, A.) Chapman and Hall, New York. Pp. 5–43.
Byong H. Lee (2015). Fundamentals of Food Biotechnology. Second edition. Wiley-Blackwell, New Jersey, United States.
Chrispeels M.J and Sadava D.E (2002). Plants, Genes, and Crop Biotechnology. 2nd edition. Jones and Bartlett Publishers, Sudbury, MA.
Clark D.P and Pazdernik N (2010). Biotechnology. First edition. Elsevier Science and Technology Books, Amsterdam, Netherlands.
Das H.K (2010). Textbook of Biotechnology. Fourth edition. Wiley edition. Wiley India Pvt, Ltd, New Delhi, India.
Dictionary of Microbiology and Molecular Biology, 3rd Edition. Paul Singleton and Diana Sainsbury. 2006, John Wiley & Sons Ltd. Canada.
Glick B.R and Pasternak J.J (2003). Molecular Biotechnology: Principles and Applications of Recombinant DNA. ASM Press, Washington DC, USA.
Godbey W.T (2014). An Introduction to Biotechnology. First edition. Woodhead Publishing, Cambridge, United Kingdom.
Jee C and Shagufta (2007). Environmental Biotechnology. APH Publishing Corporation, Darya Ganj, New Delhi, India.
Lee S.Y, Lee D.Y and Kim T.Y (2005). Systems biotechnology for strain improvement. TRENDS in Biotechnology, 23(7):349-356.
Discover more from Microbiology Class
Subscribe to get the latest posts sent to your email.