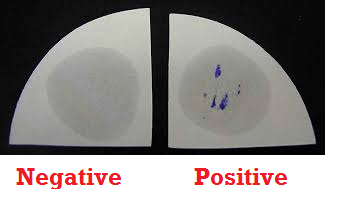
Catalase test is used to identify microorganisms that produce catalase enzyme. It is used to differentiate catalase-producing organisms (e.g. Staphylococcus species) from non-catalase producing bacteria (e.g. Streptococcus species). Catalase enzyme breakdown hydrogen peroxide (H2O2) to water and oxygen; and this leads to the release or production of bubbles – which indicates a positive test result (Figure 1).
When a culture plate containing cultures of S. aureus is flooded with H2O2, bubbles are released, and this shows that the test organism in the culture plate is S. aureus (Figure 2). The presence of catalase enzyme in the test isolate is detected using hydrogen peroxide. If the bacteria possess catalase (i.e. are catalase-positive), when a small amount of bacterial isolate is added to hydrogen peroxide, bubbles of oxygen are observed.
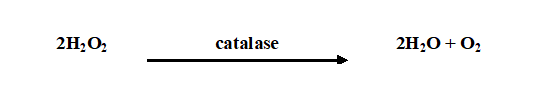
Most aerobic bacteria produce possess catalase enzyme in varying amounts except the lactic acid bacteria which do not produce the enzyme in detectable amounts. Obligate anaerobes are usually catalase negative. Hydrogen peroxide is a highly toxic product of certain cellular processes such as the reduction of flavoproteins, thus catalase test is used to determine whether or not a particular microorganism produces catalase (the enzyme that catalyzes the breakdown of H2O2). This test can be performed by two methods: the slide method and the test tube method, but only the slide method shall be expanded here.
PROCEDURE FOR SLIDE METHOD OF CATALASE TEST
- Perform this test with pure culture from culture media plate (preferably blood agar).
- Place a loopful or speck of the test organism on a clean glass slide.
- Emulsify the culture with a loopful of freshly prepared H2O2.
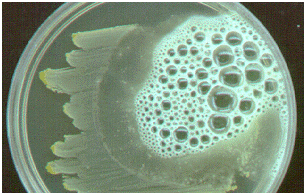
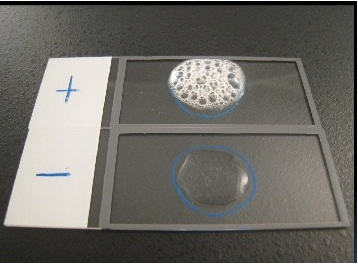
- Lookout for the release or presence of bubbles which indicates a catalase positive result. The bubbles results from the production of oxygen gas released from theaction of catalase enzyme (produced by the test organism) on the H2O2 (Figure 3).
References
Basic laboratory procedures in clinical bacteriology. World Health Organization (WHO), 1991. Available from WHO publications, 1211 Geneva, 27-Switzerland.
Beers M.H., Porter R.S., Jones T.V., Kaplan J.L and Berkwits M (2006). The Merck Manual of Diagnosis and Therapy. Eighteenth edition. Merck & Co., Inc, USA.
Biosafety in Microbiological and Biomedical Laboratories. 5th edition. U.S Department of Health and Human Services. Public Health Service. Center for Disease Control and Prevention. National Institute of Health. HHS Publication No. (CDC) 21-1112.2009.
Cheesbrough M (2010). District Laboratory Practice in Tropical Countries. Part I. 2nd edition. Cambridge University Press, UK.
Cheesbrough M (2010). District Laboratory Practice in Tropical Countries. Part 2. 2nd edition. Cambridge University Press, UK.
Collins C.H, Lyne P.M, Grange J.M and Falkinham J.O (2004). Collins and Lyne’s Microbiological Methods. Eight edition. Arnold publishers, New York, USA.
Disinfection and Sterilization. (1993). Laboratory Biosafety Manual (2nd ed., pp. 60-70). Geneva: WHO.
Garcia L.S (2010). Clinical Microbiology Procedures Handbook. Third edition. American Society of Microbiology Press, USA.
Garcia L.S (2014). Clinical Laboratory Management. First edition. American Society of Microbiology Press, USA.
Fleming, D. O., Richardson, J. H., Tulis, J. I. and Vesley, D. (eds) (1995). Laboratory Safety: Principles and practice. Washington DC: ASM press.
Dubey, R. C. and Maheshwari, D. K. (2004). Practical Microbiology. S.Chand and Company LTD, New Delhi, India.
Gillespie S.H and Bamford K.B (2012). Medical Microbiology and Infection at a glance. 4th edition. Wiley-Blackwell Publishers, UK.
Discover more from Microbiology Class
Subscribe to get the latest posts sent to your email.